

An amino acid needs to be bonded to in order to have values set for both psi and phi, for that reason the two terminal amino acids do not have values set for these angles. The orange plane is part of the between Ile and Leu (blue plane), and the angle between the blue and yellow planes is phi. The purple plane is part of the (side chains removed for clearer viewing) between Tyr and Ile (red plane), and the angle between the red and yellow planes is psi. The yellow plane serves as the references in measuring the two angles. Notice the three colored triangular planes. The initial scene shows phi and psi values for Ile. Of two other peptide bonds, and then using the technique described above identify the atoms contained in and the numerical values of the φ and ψ angles of the α-carbon connected to these two planes. that you correctly determined direction of rotation and the values of φ and ψ. After rotating the structure so that the four atoms can be clearly seen, measure and display the numerical value of ψ using the technique described above. (If the structure rotates in the course of clicking on the atoms or if you encounter some other problem, re-click on the green link 'four atoms' and restart clicking on the atoms.) The which constitute a ψ are an amide nitrogen, a carbonyl carbon, an α-carbon and a second nitrogen. After toggling off spin and rotating the structure so that you can clearly see that you are not clicking on a transparent atom, determine and display the numerical value of φ by double clicking on a carbonyl carbon of the angle, single clicking on the next two atoms and then double clicking on the second carbonly.
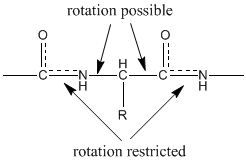
The making up φ are a carbonyl carbon, the connecting α-carbon, an amide nitrogen and the next carbonyl carbon (all marked with green halos). 1 Determining values for phi (φ) and psi (ψ)ĭetermining values for phi (φ) and psi (ψ).


 0 kommentar(er)
0 kommentar(er)
